The surprising uses of sodium carbonate (soda ash)
History
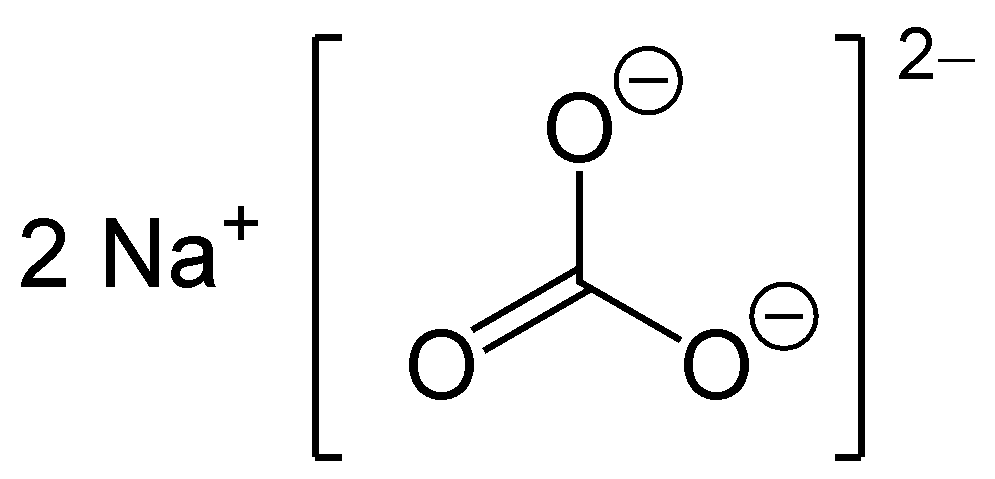
Sodium carbonate, also referred to as washing soda, soda ash, and soda crystals, is a mineral compound with various hydrated structures. All variations of this compound consist of white, odorless salts that readily dissolve in water, resulting in moderately alkaline solutions. In the past, this substance was obtained by extracting it from the ashes of plants cultivated in soils abundant in sodium. For thousands of years, humans have been familiar with and utilized soda ash. In ancient times, the Egyptians extracted this compound from a mineral called Natron, which could be found in dry lake beds.
Natron is a combination of soda ash and baking soda. The Egyptians employed soda ash in the embalming process to preserve bodies. By using this mixture, they were able to keep the deceased bodies dry and prevent decay. The effectiveness of this technique is evident in some mummified bodies that are still remarkably preserved even after 3000 years. Throughout the centuries, sodium carbonate was also derived from the burning of organic matter, particularly seaweed. This method of production is responsible for the creation of soda ash, a widely used form of this compound. However, burning deceased plants did not yield substantial quantities of sodium carbonate. As a result, chemists at the time sought out synthetic methods to produce this vital compound.
A significant breakthrough occurred in 1791 when the French chemist Nicolas LeBlanc (1742-1806) developed a technique for manufacturing sodium carbonate, which remained the industry standard for nearly a century. By 1900, the Solvay process had become the predominant method of sodium carbonate production worldwide. In the subsequent section, we will delve into the intricacies of these processes.
Methods of Production Sodium carbonate
1. Production of Sodium Carbonate from Mining:
Trona, also known as trisodium hydrogen bicarbonate dihydrate, is mined in various regions of the United State.
Large natural deposits, such as those near Green River, Wyoming, were discovered in 1938, making mining more economically viable.
Turkey has significant reserves of trona, with extraction taking place near Ankara. Dredging operations in lakes like Lake Magadi in Kenya extract sodium carbonate from continuously producing lake salt.
2. Production of Soda Ash from Plants and Algae (Barilla and Kelp):
Certain salt-tolerant plant species and seaweed can be processed to obtain the crude form of sodium carbonate.
These sources were commonly used in Europe and other regions until the early 19th century. ➢ Plants or seaweed were dried and burned, and the resulting ashes were washed with water to create an alkaline solution.
The solution was then boiled to produce soda ash, and the crude form obtained from seaweed or kelp was referred to as “Barilla.”
3. The LeBlanc Process for Sodium Carbonate Production:
- In 1792, French chemist Nicolas LeBlanc patented a process for producing sodium carbonate using salt, sulfuric acid, limestone, and coal.
- The process involved the treatment of sodium chloride with sulfuric acid in the Mannheim process, resulting in the production of sodium sulfate (salt cake) and hydrogen chloride.
- Salt cake and crushed limestone (calcium carbonate) were heated with coal, undergoing two stages of conversion.
- The first stage was the carbothermic reaction, where coal, as the carbon source, reduced sulfate to sulfide.
- The second stage involved the production of sodium carbonate and calcium sulfide.
- The resulting mixture, called black ash, was then extracted with water to obtain soda ash through evaporation.

Uses of Soda Ash
1- Glass Manufacturing Industry:
Sodium carbonate acts as a flux, raising the melting point of silica in glass production without the need for specialized materials.
It is used in the production of bottle and window glasses by melting mixtures of sodium carbonate, calcium carbonate, and silica sand.
2.Water Softening:
Sodium carbonate is used to remove temporary and permanent hardness in water.
It softens water by removing calcium and magnesium ions, which form insoluble deposits when treated with carbonate ions.
3.Food Additives and Cooking:
- Sodium carbonate has various uses in cooking, as it is stronger than baking soda but weaker than metal hydroxide alkalis.
- It affects gluten production in cooked dough and is used in the production of Japanese noodles.
- Bakers use it to create specific textures in cakes and improve browning.
- It is used as a food additive (E500) for acidity regulation, as an anticoagulant, volume agent, and stabilizer.
- In syrup powder production, the reaction between sodium carbonate and citric acid creates a cooling and tingling sensation when moistened by saliva.
Other Uses of Soda Ash:
- It is used as a strong base in various industries, preferred for its affordability and safety compared to sodium hydroxide.
- It serves as a pH regulator in photography film production.
- Soda ash is added to swimming pools and aquariums to maintain the desired pH and carbonate hardness.
- In dyeing cellulose fibers, sodium carbonate ensures appropriate chemical bonding of dyes.
- It is used in the froth flotation process as a pH adjuster and floating agent for certain minerals.
Safety
on the account of explanation of Sodium carbonate safety, provided information itemized as the following:
Sodium carbonate is non-flammable and does not have specified flammability points or restrictions.
When heated, sodium carbonate decomposes and releases NA2O smoke, but the risks of fire and explosion in the presence of various materials are not available.
- It can ignite and burn severely when in contact with fluoride.
- Soda ash decomposes in contact with fluorine at normal temperatures due to radiation.
- It reacts with hot aluminum metal.
- Sodium carbonate is generally stable, but it can become unstable in the presence of incompatible substances like acids and moisture.
- It is incompatible with substances such as phosphorus pentoxide, lithium, fluorine, fluoride, ammonia + silver nitrate, 6,4,2-tlic nitrotoluene, ammonia, acids, sodium sulfide + water, hydrogen peroxide, red metal aluminum, and sodium sulfide.
- Sodium carbonate is decomposed by acids, resulting in effervescence.
- Concentrated solutions of sodium carbonate have mild corrosive properties for steel. ➢ Polymerization does not occur with this substance.
 Storage
Storage
Regarding to storage and maintenance of the Sodium carbonate the hints below should be taken into granted.
When working with sodium carbonate, it is important to avoid inhaling the dust. Proper protective clothing, including respiratory equipment, masks, goggles, and gloves, should be worn. Avoid skin and eye contact with the compound.
Sodium carbonate packages should be stored away from incompatible materials, particularly acids. It is recommended to keep the material in a tightly closed container.
Store sodium carbonate in a cool and well-ventilated area. It should not be stored at high temperatures, and the ideal temperature for storage is around 24 °C.
Packing
Here are the noticeable items that should be informed in regard to packaging Sodium Carbonate.
- Sodium carbonate is available in the market in two main packaging options: light sodium carbonate and dense sodium carbonate.
- Light sodium carbonate is typically packaged in 50 kg bags, while dense sodium carbonate is also available in larger side bags weighing 1000 kg.
- These packaging options cater to different customer needs, allowing for convenient handling and transportation of sodium carbonate based on the order size and requirements.
Sodium Carbonate Manufacturers
Given the extensive utilization of sodium carbonate in both small and large industries, there is a significant demand for the procurement and trade of this crucial industrial substance. For those interested in purchasing sodium carbonate, one option is to consult Shimico’s product list, which includes offerings such as Maragheh sodium carbonate, Semnan sodium carbonate, and products sourced from other petrochemical or soda ash factories.
These manufacturers cater to e market’s need for acquiring this essential material. It is advisable to refer to Shimico’s available products or explore other reputable suppliers to fulfill specific sodium carbonate requirements. In brief, the important data itemized as below:
- The demand for sodium carbonate in various industries is significant.
- Shimico offers a range of sodium carbonate products.
- Maragheh sodium carbonate and Semnan sodium carbonate is available from Shimico.
- Shimico also sources sodium carbonate from other petrochemical or soda ash factories.
- It is advisable to refer to Shimico’s product list or explore other reputable suppliers for sodium carbonate procurement.